Imagine a single degree change rendering an entire shipment of life-saving medicine completely useless. In the world of pharmaceuticals, temperature isn’t just a detail; it’s the backbone of product safety and effectiveness. A minor fluctuation can transform a vital treatment into a worthless or even dangerous substance.
The industry faces unique challenges, from maintaining strict temperature ranges for delicate biologics to ensuring stability across a complex global supply chain. Failing to manage these conditions leads to significant financial losses and, more importantly, poses a serious risk to patient safety.
This guide will demystify the critical requirements for pharma cooling. We will explore the key temperature ranges, the role of specialized equipment, and the indispensable processes of validation and monitoring that ensure regulatory compliance and product integrity.
By understanding these pillars, you can build a cooling strategy that protects your products from start to finish.
Table of Contents
ToggleUnderstanding the Key Temperature Ranges
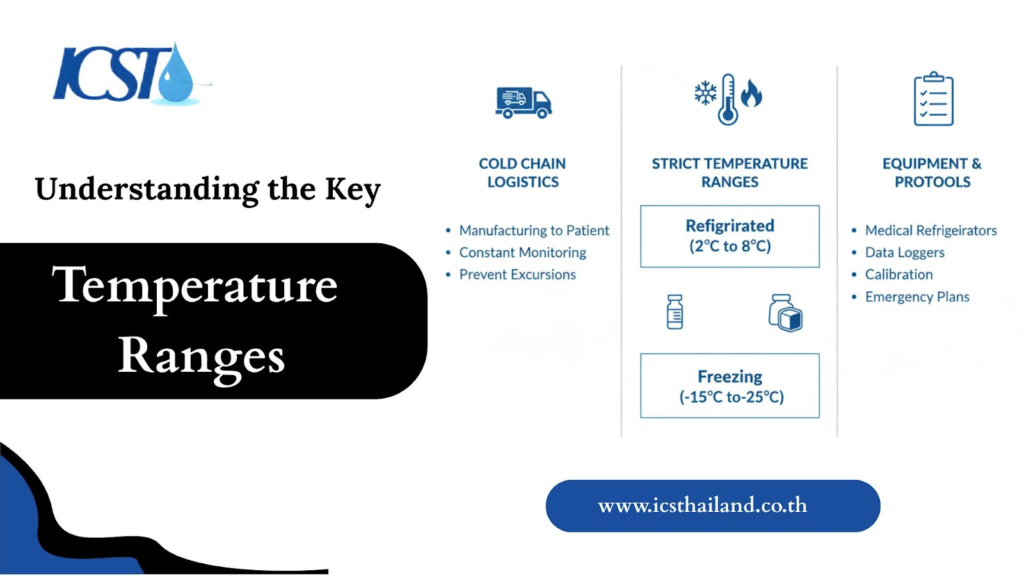
Pharmaceutical products require specific temperature ranges to maintain their chemical and biological stability. What are these critical ranges, and which products fall into each category?
Controlled Room Temperature (CRT):
This range, typically between 15°C and 25°C, is essential for most general pharmaceutical products. It protects common medications like pills and tablets from the damaging effects of excessive heat or freezing, which could alter their efficacy and shelf life.
Cold Storage / Refrigerated:
The most common “cold chain” range, from 2°C to 8°C, is vital for many sensitive biologics. This narrow window preserves the integrity of vaccines, insulin, and other protein-based therapies that would quickly degrade at room temperature.
Cryogenic / Ultra-Low Temperature:
For highly sensitive products, temperatures must drop significantly. This range, which is below -20°C and often extends to -80°C or lower, is required for certain vaccines (like some for COVID-19), advanced cell therapies, and biological samples, where it halts nearly all biological activity to ensure long-term stability.
The Foundation: GMP & GDP Compliance
Regulatory standards are the backbone of pharmaceutical temperature control. These frameworks dictate how products must be handled to guarantee their quality and safety. What do these regulations demand?
Good Manufacturing Practices (GMP)
- Good Manufacturing Practices (GMP) govern the production phase of pharmaceuticals. Their role is to ensure that the manufacturing environment is meticulously controlled to produce a consistently high-quality product.
- GMP mandates strict management of temperature and humidity within production areas and cleanrooms.
- This control is fundamental to preventing product degradation and cross-contamination, ensuring that every batch meets its specified quality attributes from the very beginning.
Good Distribution Practices (GDP)
Good Distribution Practices (GDP) are the crucial link in the pharmaceutical supply chain, ensuring that the quality and integrity meticulously built into products during manufacturing are preserved all the way to the end-user.
It is the cornerstone for maintaining pharmaceutical efficacy and patient safety outside the controlled environment of a production facility.
- Comprehensive Scope: GDP encompasses every aspect of the handling, storage, and distribution process, from the moment a product leaves the manufacturing plant until it reaches the pharmacy or patient, including receipt, warehousing, order picking, packing, and transportation.
- Maintaining Product Integrity: The primary objective is to protect pharmaceutical products from physical damage, theft, adulteration, and, critically, from temperature excursions that could compromise their stability, efficacy, and safety.
- The “Cold Chain” Mandate: For temperature-sensitive medicines, GDP rigorously defines the requirements for maintaining an unbroken “cold chain,” dictating the use of validated temperature-controlled storage facilities, vehicles, and packaging solutions.
- Risk Management and Quality Systems: It necessitates the implementation of robust quality management systems, including thorough risk assessments, deviation management, and corrective and preventive actions (CAPA) to proactively address potential issues in the supply chain.
- Traceability and Accountability: Strict documentation and record-keeping requirements ensure complete traceability of every batch, enabling rapid identification and recall of products if any quality issues arise, thereby enhancing patient safety.
- Personnel Training: Emphasizes that all personnel involved in the distribution process must be adequately trained and competent in GDP principles, understanding their role in maintaining product quality and patient safety.
Cooling Solutions for Each Stage
Meeting strict temperature requirements demands specialized equipment tailored to each phase of the pharmaceutical lifecycle. How do these solutions work to maintain compliance?
Manufacturing & Cleanroom HVAC
In a pharmaceutical manufacturing setting, standard air conditioning is insufficient. Specialized Heating, Ventilation, and Air Conditioning (HVAC) systems are required to manage not only temperature but also humidity, air pressure, and particulate matter.
These systems are engineered to meet stringent cleanroom classifications, such as ISO 7 or ISO 8, creating a stable and sterile environment.
Key equipment includes industrial chillers for process cooling, advanced air handling units (AHUs) for precise environmental control, and HEPA filtration to remove contaminants from the air.
Storage & Warehousing
- Once manufactured, products must be stored under equally controlled conditions to preserve their integrity over time.
- This requires climate-controlled warehouses equipped with robust cooling solutions. Walk-in cold rooms provide large-scale refrigerated storage, while refrigerated container units offer flexible, mobile solutions.
- For products requiring extreme cold, purpose-built ultra-low temperature freezers are essential to maintaining stability until distribution.
The LRP: Logging, Reporting, and Proof
Proving that you have maintained correct temperatures is just as important as the cooling itself. How do you document and verify compliance?
Temperature Mapping & Validation
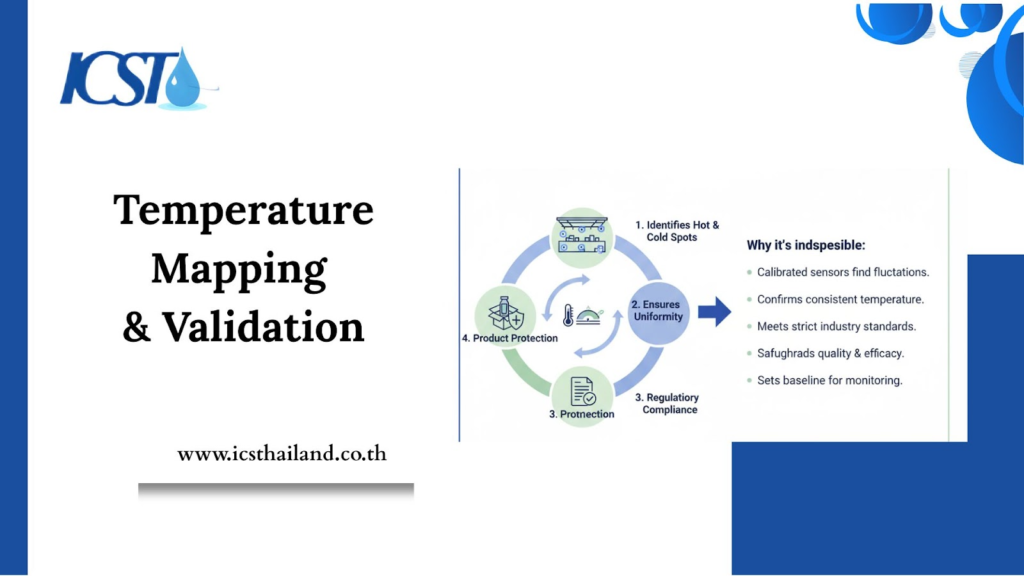
Before any storage area, such as a warehouse, cold room, or freezer, is put into use, it must undergo a critical validation process. This step is fundamental to ensuring the integrity of products sensitive to temperature.
Here’s how temperature mapping and validation work, and why they are indispensable:
- Identifies “Hot and Cold Spots”: Calibrated sensors are strategically placed throughout the storage space to detect areas where temperatures might fluctuate outside the desired range.
- Ensures Uniformity: This process confirms that the entire storage environment maintains a consistent and uniform temperature within specified limits.
- Regulatory Compliance: It provides definitive proof to regulatory bodies that the storage area is suitable for its intended purpose, meeting strict industry standards.
- Product Protection: By verifying proper temperature control, validation safeguards the quality, efficacy, and safety of the products stored within.
- Establishes Baseline: It sets an essential baseline for ongoing temperature monitoring, helping to detect any future deviations quickly.
Continuous Monitoring & Alarms
- Validation is an ongoing process, not a one-time event; temperature control demands continuous oversight.
- Automated systems with calibrated sensors offer real-time temperature data around the clock.
- These systems trigger immediate alarms upon temperature excursions, enabling quick corrective action to protect product batches.
- To ensure data reliability, these systems must comply with regulations such as FDA 21 CFR Part 11 for electronic records.
Wrap Up
Pharma cooling is a high-stakes discipline where precision and reliability are non-negotiable. It demands a deep understanding of regulatory requirements, specialized equipment, and meticulous documentation.
A robust cooling strategy, extending from the manufacturing line to final delivery, is the foundation of quality assurance and patient safety. Investing in a compliant and validated system moves your organization from simply meeting regulations to having complete confidence in your product’s integrity.
Ensure your pharmaceutical cooling system meets the highest standards for safety and compliance. For a professional consultation on a new system or to validate your existing infrastructure, contact the pharma cooling specialists at ICST today.
Frequently Asked Question
What are the key temperature ranges for pharma products?
The key ranges are Controlled Room Temperature (CRT) at 15°C to 25°C, Cold Storage at 2°C to 8°C, and Cryogenic/Ultra-Low Temperature below –20°C.
What is the difference between GMP and GDP?
GMP (Good Manufacturing Practices) governs temperature control during the production phase, while GDP (Good Distribution Practices) ensures product integrity during storage and transportation.
What is the “cold chain”?
The cold chain is a rigorously defined process of maintaining a specific temperature range for a product from the moment it leaves the manufacturing plant until it reaches the end-user.
Why is temperature mapping important?
Temperature mapping is a critical validation process that uses strategically placed sensors to identify “hot and cold spots” in a storage area, ensuring the entire space maintains a uniform temperature for compliance and product protection.
Why is continuous monitoring a requirement?
Continuous monitoring provides real-time data and triggers immediate alarms upon temperature excursions, allowing for quick corrective action to protect product batches and ensure data integrity.